Products exported to 30 countries
Seohancare has been a leading manufacturer of a range of orthopedic and neurosurgical implants in Korea since 2005. Seohancare's products have been used effectively for many surgical products.
The Company exports 20 medical devices to 30 countries, including Taiwan, Thailand, Australia, Iran, Saudi, Egypt, Russia, Italy, Belgium, Spain, Greece, Turkey, the UK, Portugal, Ukraine, Czech, Mexico, Brazil, Colombia and Argentine.

SeohanCare is working tirelessly to develop a range of innovative products and manufacturing technologies for fair cost, but high quality. The Company imports only top-quality ingredients from USA, the UK, Germany and other countries to produce premium medical devices. The Company is headquartered in Gimpo, Gyeonggi Province. The Company has the Seoul Office and Gwangju Factory.
Seohancare ’s father company is SSB TECH, a driving system manufacturer who has localized power and driving transfer systems. The company manufactures and provides a range of semiconductor, LCD and robot parts to Korea’s leading electronics company such as Samsung Electronics, LG Electronics and SK Hynix.
Seohancare ’s Vision is a “Worldwide Company of Medical Devices for Better Life Quality.”
Seohancare possesses several cutting-edge technologies which follow:
- Ceramic Precision Engineering Technology
- Titanium Forging Technology
- Bio Polymer (PEEK/PLA,PGA)Injection Molding Technology
- Plasma/RF Device Technology
- Robotic Surgery Technology
◇ The product line includes:
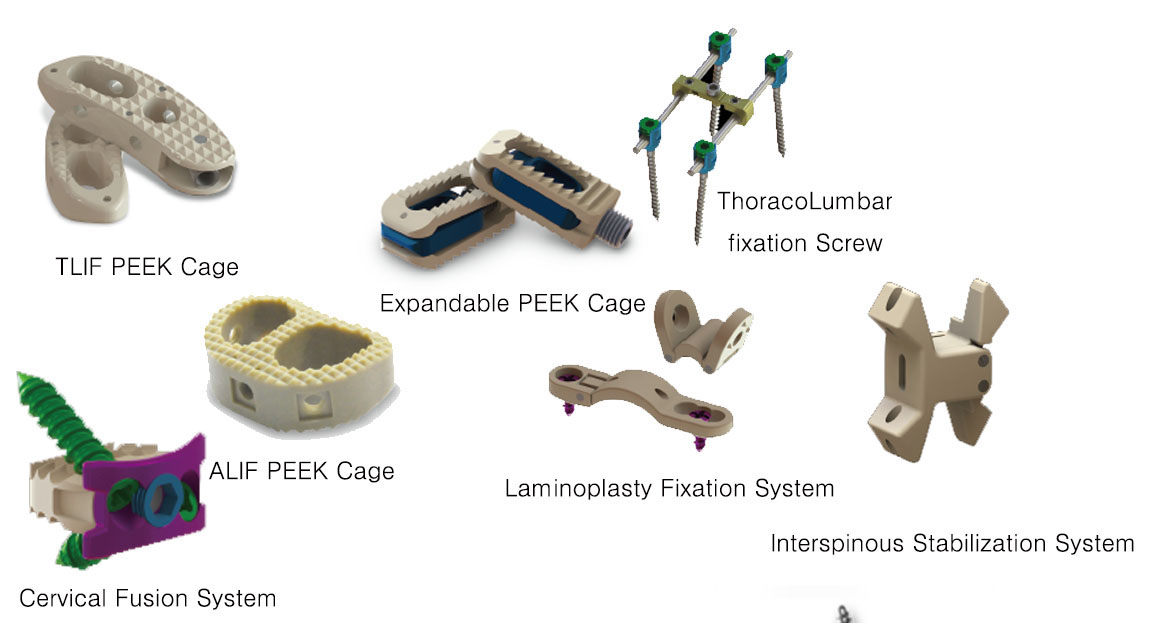
ACIF PEEK CAGE
Feature
-The entire geometry is taper wedged with the surface. And these teeth are contra-direction against prolapse for preventing from toothed, the superior and inferior of
-Teeth on superior and inferior faces resist implant migration
-Composed of PEEK-Optima, a material with a proven clinical history that provides structural strength
CERVICAL FUSION SYSTEM
Feature
-Angled teeth treads guide the implant into position and resist migration
-Large graft areas for maximum fusion potential
-Bullet-shaped tip aids in distraction and insertion
-Curved implant shape matches contour of vertebral body
PLIF PEEK CAGE
Feature
-The entire geometry is rectangular and the superior and inferior of its surface are toothed and these teeth are contra-direction against prolapse for preventing prolapsed or dislocation.
-Anterior part is round to easy to inset it and has several angles of O, 4 and 8 degrees so that it can enable patients to keep the spinal curve.
-Toothed surface anti-backout
-It is punctured on its center into which you can insert the patient’s own one of synthetic bone to induce and improve the intervertebral fusion.
PEDICLE SCREW
Feature
-Consists of four components: The screw shank, the tulip portion of the screw and two internal locking rings.
-The screw shank has four-prong drive interface with a “proprietary peak” created the center of the four-prolong drive, which significantly enhances the axial grip on the longitudinal length of the road.
LLIF PEEK CAGE
Feature
-The cage and its anchoring systems are directly inserted along the axis of the disc allowing for a minimal invasive lateral approach to the spine, reducing vascular risks and respecting anatomic structures.
-Half anchoring plates are preassembled on a single-use anchoring plate holder made of PEEK Optima avoiding any direct manipulation and simplifying insertion.

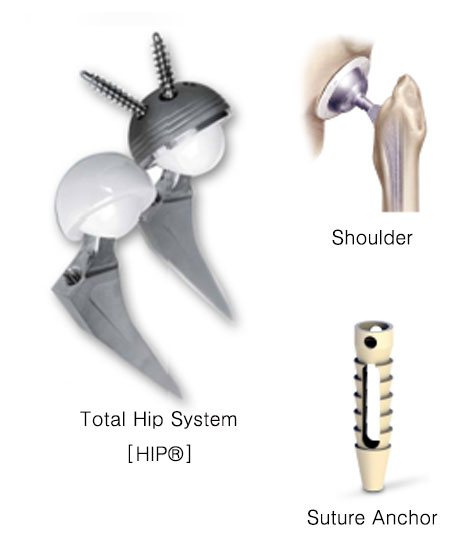
HIP
-Titanium plasma spray porous coating indices early osseo integration and quickly initial fixation.
-Hybrid zirconia ceramic has superior properties and wear resistance than alumina ceramic
-Ceramic on ceramic system shows the lowest wear rates. Paper fixation prevents the dislocation and micromotion. Tight clearance promotes stable fixation.

◇ Company History
2016.01 Performed the technical innovation development project of the Small and Medium Business Administration
(Developing localization of ceramic artificial hip joint)
2015.08 Developed HCS, Interference Screw using PEEK material
2015.07 Localization of Suture Anchor using PEEK material
2015.07 Exported 30 countries and Acquisition of Brazilian Anvisa.
2014.06 Spinal Fixation Implant (MEGAFIX) KFDA & CE approved
05 Fracture joint fixed implant (Trauma Plate) KFDA & CE approved
Cervical union system (Perfect-C) KFDA & CE approved
2013.09 First in Korea to get an approval of KFDA for hybrid Expandable Cage. KFDA approval for minimal invasion TLIF PEEK Cage.
.07 Selected as a small and medium-sized innovation company (INNO-BIZ)
.06 Technology innovation development project of small and medium-sized enterprises
(development of stand-alone type DLIF cage and spinning device)
2012.12 Selected as a small business export certification company
10 Acquisition of stabilization implant CE mark (DNV) between sprockets
05 Vertebral Lacrimal Arthroplasty Fixed Implant KFDA approved
03 KFDA approval of Stabilized Implant between spinous process.
2010.10 Gyeonggi-do Technology Development Project performed
(Project name: Interspinous stabilization system)
2009.10 Started exporting to 5countries including Russia, Iran and Belgium.
2008.07 Acquired the CE mark (DNV) of ISO13485and PEEK chin-arch fusion bead for the first lumbar vertebra in Korea
.06 Achieved KFDA / GMP certification for Korea's first localization of PEEK (ALIF) interbody fusion splint for cervical vertebra
.03 Achieved KFDA / GMP certification for Korea's first localization of PEEK (PLIF) interbody fusion splint for cervical vertebra
.01 Establishment of affiliated research institute
2007.02 Registered as a venture company
2005.10 First in Korea to contract an agreement with Invibio Ltd. for bio-polymer PEEK Optima
.01 Development of Ball & Liner for Hip
.01 Established Seohancare Co., Ltd.

